Local Label Solutions – 3 New England Producers

From specialty foods, to craft beer and hemp producers, we’ve teamed up with some of the areas most well-known companies, delivering custom label solutions that stick. When it comes to labels and packaging, every business has its own unique set of challenges. We’ve picked up our fair share of tricks along the way and our […]
Cheese Labels: A Producers Guide to Labeling

Cheese labeling has it’s own unique set of specifications set forth by the Department of Food Safety in the state of origin. Producers are challenged with not only creating a label that provides brand identity and shelf appeal but also contains all the necessary components of a proper nutrition label. By following these guidelines, you will […]
ImageTek Labels Receives ISO 9001: 2015 Certification for Quality Management Systems

Recognized for the manufacturing and distribution of printed media, blank label stock, print ribbons, lamination supplies and custom printed media, the certification is evidence of the value we place on customer relationships, and our dedication towards continuous improvement. ISO 9001: 2015 certification allows us to maintain mutually beneficial partnerships with global manufacturing professionals looking for durable, custom label […]
Wine And Glass Bottle Labels: Choosing A Suitable Media

Label Wine and Glass Bottles for Special Occasions Whether you’re a wine enthusiast looking to bottle your own blend or just wanting to create personalized glass bottle labels for a wedding or social event, you want to choose the right label stock for a finished look that is just right. Though there are lots of different types […]
ImageTek Labels to Attend MFG4

Manufacturing for the Future Exposition in Hartford, CT. May 3 – 5, 2016 – Visit Us at Booth #1817 DOWNLOAD A COMPLIMENTARY GUEST PASS HERE! Just tell them you’re with ImageTek Labels (wink, wink). MFG4 for Aerospace, Industrial and Military Defense With today’s ever evolving manufacturing climate, technology is constantly adapting and changing… moving towards custom, […]
FDA Approves Proposed Medical Device Labeling
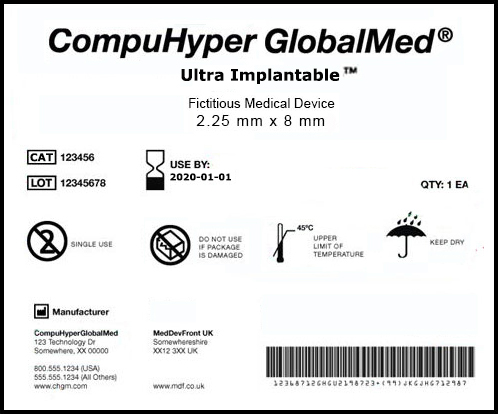
Are You Prepared for the FDA’s Medical Device Labeling Regulations? The Food & Drug Administration (FDA) has announced the final ruling that will require medical device manufacturers to label each unique medical device distributed in the U.S. with a UDI “Unique Device Identifier” label. Are you prepared for the FDA’s medical device labeling regulations? The […]
UL969/IEC60601-1 3rd Addition for Certification for Medical Device Labeling

New UL/IEC 60601-1 3rd edition regulations have been extended until Dec 31st, 2013 New regulations proposed by the FDA are in the final stage rulings. The new legislation affects over 25,000 medical device manufacturers worldwide. Are you one of them? Beginning in 2014, manufacturers of most medical devices will be required to mark all manufactured […]
